
Education
- Ph.D. Chemistry, Rice University, Houston, TX (w. R. E. Smalley)
- M.S. Chemistry, Emory University, Atlanta, GA
- B.S. Chemistry, Xiamen University, China
Professional Experience
- Professor, University of Maryland, 8/2017 – present
- Associate Professor with tenure, University of Maryland, 8/2-14 – 7/2017
- Assistant Professor of Chemistry, University of Maryland, College Park, fall 8/2008 – 7/2014
- Faculty member, Chemical Physics Program, University of Maryland, 12/2008-present
- Faculty member, Maryland NanoCenter, 8/2008 – present
- Postdoctoral Associate, Northwestern University, 2005-2008 (w. Chad A. Mirkin)
- Project Leader, Carbon Nanotechnology Laboratory, Rice University, 2001-2004
Research Interests
Organic color centers, quantum defect chemistry, quantum sensing, energy science, carbon nanotechnology.
Major Recognitions and Honors
- Life Sciences Invention of the Year Award, University of Maryland, 2023
- Fellow, The American Physical Society (elected, 2021)
- The Kirwan Faculty Research and Scholarship Prize, 2019
- Honoree, Maryland Research Excellence Celebration, Office of the Vice President for Research, University of Maryland, 2019
- NBC4 News AAPI Working for the Community Award, 2019
- Washingtonian Magazine's Guest List: 5 People We'd Love to Hang Out With This April, 2019
- Department of Energy EFRC Ten at Ten People Awards Nominee, NEES EFRC 2019
- NSF CAREER Award in Chemistry, 2011-2016
- Invention of the Year finalist, University of Maryland, 2011
- ACS Petroleum Research Fund Doctoral New Investigator, 2010
- Alumni Spotlight, the Wiess School of Natural Sciences, Rice University, 2010
- Nanobiotechnology Award, Maryland Department of Business & Economic Development, 2009
- General Research Board Research Support Award, University of Maryland, 2009
- General Research Board Summer Award, University of Maryland, 2009
- NSF NSEC Outstanding Researcher Award, Northwestern University, 2007
- David G. Nance Award for Outstanding Graduate Research in Nanotechnology, Texas 2004
- The Presidential Fellowship, Rice University, 2000–2004
- Robert A. Welch Foundation Predoctoral Fellowship, 2000–2001
Significant Professional Service and Activities
- Lead Organizer, B03 Symposium, Carbon Nanotubes --From Fundamentals to Devices. ECS National Meetings (2025--)
- Co-organizer, Carbon Nanotubes --From Fundamentals to Devices. 245th ECS Meeting, San Francisco, CA, United States. May 26-30, 2024.
- Technical Abstract Review Committee, TechConnect World Innovation Conference, Washington, DC (2020-)
- Organizer and Host, CENT2 EFRC Kickoff Symposium, University of Maryland, College Park, August 17-18, 2023
- Advisory Board: International Conference on the Science and Application of Nanotubes and Low-dimensional Materials (2019-2023)
- Organizer and Host, “The Kickoff Meeting of the Center for Enhanced Nanofluidic Transport (CENT), a DOE Energy Frontier Research Center. November 16, 2018. University of Maryland, College Park, Maryland.
- Workshop Co-Organizer, “Telluride Workshop on the Defect Chemistry and Physics of Low-dimensional Materials,” July 11-15, 2017 (co-organizer: Phil Collins). Telluride, Colorado.
- Symposium Co-Organizer, “At the Interface Between Molecules and Materials.” The American Physical Society March Meeting, March 2-6, 2015. San Antonio, TX. (w/ George C. Schatz, Emily Weiss).
- Symposium Organizer, “Symposium on Energy Materials.” May 16, 2014. College Park, Maryland.
- Workshop Co-Organizer, Telluride Workshop on the Chemistry and Physics of Defects in Carbon Nanotubes, Telluride, Colorado. July 8-12, 2013 (co-organizer: Phil Collins)
- Vice-Chair of Organizing Committee, “7th Sino-US Forum on Nanoscience and Nanotechnology.” June 8-11, 2012. Xiamen, China.
- Symposium Organizer. “Chemistry of Carbon Nanomaterials,” the Middle Atlantic Regional Meeting of the American Chemical Society, May 21-24, 2011.
- Symposium Organizer. “Chemical Methods of Nanofabrication,” 237th ACS National Meeting, March 22-26, 2009. This three-day symposium featured 25 invited talks under the Salt Lake City meeting theme, “Nanoscience: Challenges for the Future.” (co-organizers: Chad Mirkin, So-Jung Park)
PUBLICATIONS
https://scholar.google.com/citations?hl=en&user=2hZ1hewAAAAJ
Quantum Defects, Tube^2, E=mC^2 & Beyond
The Wang research group focuses on Materials innovation, Chemical manipulation, Carbon nanotechnology and beyond (E=mC^2 & beyond). We synthetically create new materials with exciting new properties from low-dimensional carbon materials. New chemistries and tools are being developed in our laboratory to directly tailor nanostructures at the electronic and optical levels. In so doing, we have been able to open new research directions and develop technology platforms for discovering new phenomena and properties at both the very small length scale and nanostructured interfaces that may allow extending nano to the very large length scale. Examples of our current research include creating molecularly tunable fluorescent quantum defects to understand and control the coupling of electrons, excitons, phonons, and spin with defects in reduced dimensions, establishing the molecular science of carbon, elucidating the fundamental principles that govern the assembly and collective properties of nanostructured solids and networks, and developing novel devices, instrumentation and methodologies to tackle some of the most fundamental problems in energy, nanofabrication, quantum science, electronics, and biomedicine.
1. Molecularly Tunable Fluorescent Quantum Defects.
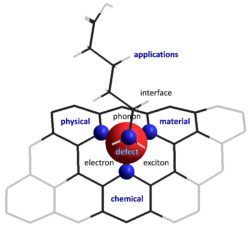 Defects can rule the properties of a crystal. This effect is particularly intriguing in atom-thick materials such as single-walled carbon nanotubes (SWCNTs) and graphene, where new chemistry and physics may arise due to strong coupling of electrons, excitons, phonons, and spins with defects in reduced dimensions. Our lab has synthetically created an entirely new class of defects that we call molecularly tunable fluorescent quantum defects, or quantum defects for short. We are exploring these chemically tailored, fluorescent quantum defects as a new toolkit for materials engineering, for brightening dark excitons, for creating near-infrared quantum emitters and single photon sources, and for probing chemical events that could be otherwise difficult to capture.
Defects can rule the properties of a crystal. This effect is particularly intriguing in atom-thick materials such as single-walled carbon nanotubes (SWCNTs) and graphene, where new chemistry and physics may arise due to strong coupling of electrons, excitons, phonons, and spins with defects in reduced dimensions. Our lab has synthetically created an entirely new class of defects that we call molecularly tunable fluorescent quantum defects, or quantum defects for short. We are exploring these chemically tailored, fluorescent quantum defects as a new toolkit for materials engineering, for brightening dark excitons, for creating near-infrared quantum emitters and single photon sources, and for probing chemical events that could be otherwise difficult to capture.
2. Tube^2.
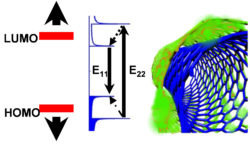 Atom-thick materials such as SWCNTs and graphene are prone to chemical attack because all of the constituent atoms are exposed. To overcome this materials limitation, my research group has synthesized a tube-in-a-tube (Tube^2) semiconductor that is equivalent to a SWCNT hermetically sealed by a chemically tailored outer wall, created from double-walled carbon nanotubes through outer wall-selective covalent chemistry. This concept allows us to address a series of fundamental questions central to nanomaterials chemistry. For example, how are the electronic properties of a semiconducting nanostructure affected by its chemical environment? What if the environmental effects could be controlled or completely eliminated by protecting the inner-tube with the outer wall? Will electrical transport in such a “double wall” structure become immune to chemical perturbations by oxygen and water? Will it fluoresce brightly?
Atom-thick materials such as SWCNTs and graphene are prone to chemical attack because all of the constituent atoms are exposed. To overcome this materials limitation, my research group has synthesized a tube-in-a-tube (Tube^2) semiconductor that is equivalent to a SWCNT hermetically sealed by a chemically tailored outer wall, created from double-walled carbon nanotubes through outer wall-selective covalent chemistry. This concept allows us to address a series of fundamental questions central to nanomaterials chemistry. For example, how are the electronic properties of a semiconducting nanostructure affected by its chemical environment? What if the environmental effects could be controlled or completely eliminated by protecting the inner-tube with the outer wall? Will electrical transport in such a “double wall” structure become immune to chemical perturbations by oxygen and water? Will it fluoresce brightly?
3. Nanoelectrode Networks for Energy Storage and Harvesting.
Owing to their high conductivity and current-carrying capacity, carbon nanotubes may vastly improve energy harvest, transport, and storage. The projects in our lab currently focus on studying electrical transport through nanotube interfaces, with a goal of harnessing the collective properties of nanostructures, and synthesizing heterogeneous nanostructures that are electrochemically reversible as the anode of lithium ion batteries. The remarkable electron accepting capabilities and charge transport properties of SWCNTs have also suggested new possibilities for overcoming the efficiency bottleneck currently facing several next-generation solar cells.
4. Question Y.
The researchers in the Wang group like to ask impossible questions, and we are always seeking the wow moments. Stay tuned.


